From Jake Fray, MLS, Denver Health POC Coordinator via Joan Polancic, MLS, Denver Health School of Medical Laboratory Science.
We are having major issues with our point of care Coaguchek devices not correlating with our main lab Stago results.The pharmacists that use the Coagucheks for their outpatient anti-coag testing are getting pretty upset that there is such a difference between the two methods and they are not sure which one to base treatment. Do any of you know of another lab that uses the Stago analyzer and has POC anti-coag devices? I’m trying to gather information about what they do for the required 6 month method comparisons. Thanks! Jake.
Jake adds: There is a high bias on the part of the Coaguchek when compared to the Stago that seems to get bigger at the INR increases. We used to assign the cap cut-off of the Coaguchek as INRs greater than 4.0, which are sent to the lab for testing and the patient would be dosed off of the lab results. However, during my last method comparison I had multiple failures when the INR is greater than 2.8 comparing the Coaguchek to the Stago. CLIA states that INR values should be within 0.5 INR and I had at least one failure out of five tests per site. We know that they are different methodologies, different ISI between the two platforms, Coagucheck uses human thromboplastin where Stago uses rabbit, etc. My last method comparison these were the case as well and it still managed to pass even with readings in the 3.0–4.0 INR range. The only change that has happened since the last comparison was a bulletin that was sent out by Roche saying they increased their levels of a chemical component called Polybrene in the test strips. They said this won’t affect patient results but it is the only thing that has changed this year. I still have the same operators, same devices, QC passes every time, same patient population etc.
George sent Jake’s question to Paul Riley, PhD, who manages validation issues for Stago. Dr. Riley contacted Jake directly to work on the issue.
George also contacted colleague Dave McGlasson, MLS, who has performed several POC to plasma PT correlations. His response is…
In two different studies I conducted for our Coumadin clinic at JBSA Lackland, 59th Clinical Research Division, we found that as the INR rises, the more inaccurate the equation becomes with both the CoaguChek and the ISTAT. When we performed these studies, anything above an INR of 4.0 on the POC system was checked against the Stago STAR-Evolution. In any system we have found that the higher the INR the more inaccurate it becomes. See: McGlasson DL, Romick BG, Rubal BL. Comparison of a chromogenic factor X assay with international normalized ratio for monitoring oral anticoagulation therapy. Blood Coag Fibrinolysis 2008;19:513–7. And also: Unreliability of international normalized ratio for monitoring warfarin therapy in patients with lupus anticoagulant. Rosborough TK, Shepherd MF. Pharmacotherapy 2004; 24::838–42.
Dave has also found that a mechanical clot detection system matches more closely than the optical clot detection system. So the comparison of the reagent-instrument combinations plays a huge part in determining the accuracy of the INR end-point.
Dave further adds that in the therapeutic range INR of 2.0-3.0 there were no statistical or clinical differences between the Coaguchek and the STAR-Evolution system when using Neoplastine CI+ with a low ISI. The problems always occurred when the INRs were above the therapeutic range. McGlasson DL A comparison of INRs after local calibration of thromboplastin international sensitivity indexes. Clin Lab Sci 2002:15:91-5.
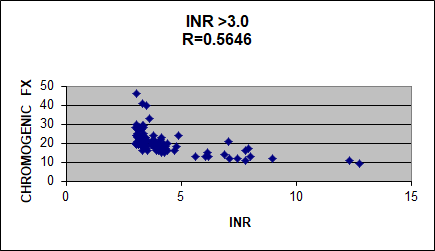
Dave added: George I found a curve that represents the chromogenic factor X (CFX) assay results from patient plasmas with INRs above 3. Look at how poorly the CFX and INR correlate. Above 3, the INR always flattens out. I conclude the INR is an inadequate way to monitor Coumadin.
I (Geo) look forward to additional contributions to this provocative topic.


Hi , just for interest I
Hi, just for interest I reviewed some 2010 data when we compared Stago CI Plus on the STAR vs CoagUChek. A POC INR of 3.5 became our caution limit. This same trend was demonstrated over numerous strip lots. The relationship changed when we changed to a recombinant thromboplastin in the lab. Robyn (SNP – Australia)
[From Geo: thank you Robyn, it seems like many of us use 4.0 as our action limit. I’m curious how the data came out with the recombinant thromboplastin.]